Dmitri Mendeleev, a great scientist and the founder of the periodic table

 Dmitri Mendeleev
Dmitri MendeleevWe all have undoubtedly heard the name of Mendeleev's chemical table of elements, the founder of this table was a Russian chemist named Dmitri Mendeleev Was. He was able to predict a large number of elements that had not yet been discovered.
An abstract of Dmitri Mendeleev's biography:
Full name: Dmitri Ivanovich Mendeleev
Date of birth: February 8, 1834
Place of birth: Verhnie Aremzyani village, near Tobolsk, Russian Empire
Nationality: Russian
Profession: Chemist
Fame: Foundation of Mendeleev's table of chemical elements
Place of study: Saint Petersburg State University
Awards: Davy Medal (1882), Fellow of the Royal Society (1892), Copley Medal (1905)
Died: February 2, 1907 (age 72), Saint Petersburg, Russian Empire
 Biography of Dmitri Mendeleev
Biography of Dmitri MendeleevBiography of Dmitri Mendeleev:
Dmitri Ivanovich Mendeleev was born on February 8, 1834 in a village called Verhnie Aremzyani, near Tobolsk in the Russian Empire. He grew up in an average but large family as the fourth child of his father. His father's name was Ivan, who worked as a director in one of the local schools, his mother's name was Maria, and she worked in the glass workshop that she inherited from her father in order to support her husband. Mendeleev's grandfather also worked as the head of the first local newspaper in Siberia.
Dmitri Mendeleev lived a quiet and good life until his father died of heart disease and he became an orphan. After that, sadness and despair filled the entire atmosphere of their home, and Mendeleev, who was no more than 5 years old, was depressed over the loss of his father.
His mother worked more to support her large family. He was working day and night in the glass workshop to make all kinds of glass containers and to keep his children comfortable and earn their education fees.
Dmitri Mendeleev went to school in Topolsk and was able to show his brilliant talent in mathematics and physics to his teachers. In the evenings, when he finished school, he would go to his mother's side to help her make glass. His uncle Besargin was a good guide and friend for this little scientist. When Dmitri was 14 years old, his mother promised him to send him to a school in St. Petersburg to continue his education, but luck was not so kind to him and his mother's glass workshop caught fire and all their capital went up in smoke.
In order to find a lucrative job, Mendeleev went to St. Petersburg and started teaching in a school. In 1850, he was able to receive a scholarship and continued his studies in mathematics, physics and chemistry. After his condition improved, he brought his family to St. Petersburg, but he lost his mother and sister due to tuberculosis, and again the shadow of sadness covered Dmitri's life.
 Gold medal of Dmitri Mendeleev
Gold medal of Dmitri MendeleevMendeleev's brilliance in the university:
Although the problems and pressures of Mendeleev's life were many, he did not abandon his studies and passed university courses one after another with excellent grades. Mendeleev became ill due to extreme poverty and grief, the condition was so bad that the doctors thought he had tuberculosis, so they advised him to go somewhere with good climate and get some rest. He went to the Crimean islands and his health gradually improved and he returned to St. Petersburg.
A. Vaskersenka, the great Russian chemist, taught chemistry to this scientist, and in 1855 he was able to receive a gold medal in this field and graduate. After that, he started teaching in high school and published the book of organic chemistry, which was considered the first textbook of organic chemistry in Russia. Dmitri Mendeleev was invited to France and Germany to participate in the scientific conference.
He published a book titled “Union of Water and Alcohol” in the field of industrial chemistry and also received his doctorate and was able to become a professor of chemistry at the University of St. Petersburg. He published several books titled inorganic chemistry and principles of chemistry, which attracted the attention of chemistry professors.
In 1864, he met and married a girl named Fezvoz Leshua at the university. The result of this marriage were two children, a boy and a girl named Volodya and Olga. But this marriage did not end well and led to divorce and separation.
 Dmitri Mendeleev's youth
Dmitri Mendeleev's youthEstablishing the periodic table:
In 1863, the sum of the elements that were found at that time at an approximate rate of one element per year; Their total number reached 56. In 1865, John Newlands paid attention to the relative weight of atoms at the same time as he published the description of the Octave Law. This led to the discovery of some other new elements such as Germanium. This concept was criticized and his innovation was not recognized by scientists until 1887. Lothar Mayer, in a writing in 1864, estimated 28 potential elements according to their chemical capacity.
But Mendeleev's table predicted the existence of 92 elements, which had no other fans except for Lothar Mayer, who published a table similar to Mendeleev's one year after Mendeleev's. Mendeleev's seemingly strange predictions about the existence of elements were often cited as examples in chemistry books, and there are few chemistry books that do not mention the titles “Eka Aluminum”, “Ekabor” and “Ekasilisium”, of course These elements were later (after discovery) known as gallium, scandium germanium.
Among the three elements predicted by Mendeleev, “Acacilium” (germanium) was discovered after the rest of them in 1887, and the discovery of the other two elements was mostly due to luck and coincidence.
In fact, the discovery of gallium was done by Boisbaudran in 1875 through spectroscopic method, and the separation of scandium was done by Nielson and Cleo in 1879, and it is related to the detailed investigation of rare earths, which was at its peak at that time.
Gradually, all the predictions made by Mendeleev came true, and the final confirmation was also received by the specific weight of metal scandium. In 1937, Fischer, who was a German chemist, was able to prepare scandium with a purity of 98%. Its specific gravity was three grams per cubic centimeter, which is exactly what Mendeleev predicted.
In the fall of 1879, Engels obtained a comprehensive book, the authors of which were Roscoe and Schorlemmer. In that book, Mendeleev's predictions of “eca-aluminium” and its discovery under the name gallium were mentioned for the first time. In an article that was later published by Engels, Mendeleev mentioned this article and concluded that:
“By unconsciously applying Hegel's law of converting quantity into quality, Mendeleev realized a scientific fact that, according to Tehor, was only comparable to Laurie's work in calculating the orbit of the unknown planet Neptune.”
When argon and helium were discovered in 1894 and William Ramsay used Mendeleev's table to predict the existence of the elements neon, krypton and xenon, Dmitri Mendeleev's name became famous. It was during these years that all the academies of the countries of the world invited him to be a member.
Since Mendeleev proposed the periodic table, most of its houses were empty, but elements were discovered one after another, and the last house was discovered in 1938, named Actinum in Paris.
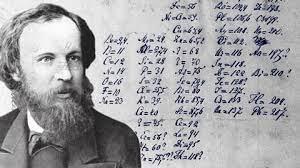 Calculations left by Dmitri Mendeleev
Calculations left by Dmitri MendeleevOther achievements of Dmitri Mendeleev:
Mendeleev's studies and achievements were not limited to the periodic table, this scientist made a major contribution to chemistry. Lev Chugaev; Russian chemist and historian of science from Mendeleev as a genius chemist, a first-rate physicist, a prolific researcher in the fields of hydrodynamics, meteorology, geology, some fields of chemical technology such as explosives, oil and fuel; and other subjects close to physics and chemistry; An expert in the chemical industry and an expert in the industry in general, as well as an original thinker in economics.
Mendeleev was also active in agricultural sciences and he also presented hypotheses about ether. Regarding the studies he had in the field of physical chemistry, he investigated the expansion of liquids with heat and obtained a relationship similar to Gilsac's law regarding the uniformity of the expansion of gases.
He investigated the origin of oil and the result was that hydrocarbons are created deep in the earth. He studied the composition of oil and contributed to the construction of the first oil refinery in Russia.
He paid attention to the importance of oil as a source of oil derivatives and materials obtained from it. It is his famous interpretation that burning oil as fuel is “like lighting a gas stove with bills”.
 The great scientist Dmitri Mendeleev
The great scientist Dmitri MendeleevPrivate life of Dmitri Mendeleev:
Mendeleev married twice in 1862 and 1882. His first marriage ended in separation, but the result was two children, a girl and a boy, although Mendeleev also had two boys and a girl from his second wife.
 Statue of Dmitri Mendeleev
Statue of Dmitri MendeleevDeath of Dmitri Mendeleev:
This scientist, who had no hope of surviving at the age of 21, died of pneumonia in 1907 when he was 73 years old. When he died, 86 elements of the periodic table had been discovered.
Today, however, this number has reached 92, and man is trying to create new elements by bombarding atoms. Element number 101 of this table is known as “mendelium” element.
compilation: Cover biographical section






